The acentric factor is defined as a function of the critical temperature, critical pressure and vapor pressure. The Lee + Kesler Relation can estimate the vapor pressure given values for the normal boiling point, the critical temperature and the critical pressure. Thus, combining this vapor pressure estimation method with the definition of the acentric factor enables us to estimate values for the acentric factor using these same three input physical properties.
The estimation code contains four main steps:
- the normal boiling point is retrieved or estimated (code lines 012 through 015)
- the critical temperature is retrieved or estimated (code lines 017 through 020)
- the critical pressure is retrieved or estimated (code lines 022 through 028 - note the technique requires the critical pressure be in the non-standard units of atmospheres)
- these values are inserted into the technique's final calculations (code lines 045 through 054)
References:
- Robert C. Reid, John M. Prausnitz and Bruce E. Poling. "The Properties of Gases and Liquids." McGraw-Hill Book Company. New York, New York, USA. Edition 4, 1987.
Being an equation-based technique, the Lee + Kesler Relation requires values for input physical properties. Specifically, property values are required for the normal boiling point, critical temperature, and critical pressure.
For example, values for the required properties of 3-isopropyl-6-methylene-1-cyclohexene are given in the table to the right. Note that each of these values was estimated by a group contribution technique.
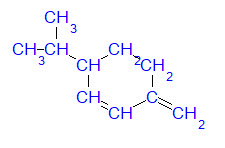
Inserting these property values into the model shown at the top of the page yields an estimate of
| Example Calculation | |||
|---|---|---|---|
| Property | Ref | Value | Units |
| Boiling Point | 1 | 431.76 | K |
| Critical Temperature | 2 | 630.74 | K |
| Critical Pressure | 3 | 27.54 | atm |
| Numerator | --- | -0.8876 | --- |
| Denominator | --- | -2.5143 | --- |
| Estimate | --- | 0.3530 | --- |
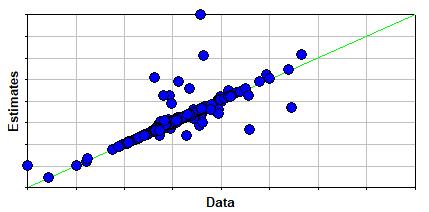
The figure to the right shows good agreement between estimates and data values. The two outliers, located in the graph's top-middle section, are o-terphenyl and m-terphenyl, were excluded from the calculation of the summary statistics. Note that as an equation-oriented technique, estimation errors may be caused by errors in the input physical properties.
This evaluation was performed on January 31, 2023 using Cranium, Professional Edition version 5.0.
| Summary Statistics | ||
|---|---|---|
| Statistic | Value | Units |
| # Observations | 314 | - - - |
| Avg Abs % Error | 12.677 | % |
| Max Abs % Error | 930.2 | % |
| Min Abs % Error | 0.070346 | % |
| Avg Abs Error | 0.028767 | - - - |
| Max Abs Error | 0.63288 | - - - |
| Min Abs Error | 0.000068148 | - - - |
| Avg Error | 0.014307 | - - - |
| Max Error | 0.63288 | - - - |
| Min Error | -0.47644 | - - - |
| Example Estimation Errors | |||
|---|---|---|---|
| Chemical | Data [K] | Estimates [K] | % Error |
| Toluene | 0.26 | 0.28 | 4.624 |
| Ethyl acetate | 0.36 | 0.38 | 4.152 |
| Benzene | 0.21 | 0.22 | 4.491 |
| o-Xylene | 0.31 | 0.32 | 4.455 |
| Benzaldehyde | 0.32 | 0.34 | 8.272 |
| Isopropylbenzene | 0.33 | 0.34 | 4.856 |
| Cyclohexanone | 0.45 | 0.29 | -34.521 |
| Hexamethyldisiloxane | 0.43 | 0.43 | -0.947 |
| Ethanethiol | 0.19 | 0.20 | 4.136 |
| Cyclohexanol | 0.53 | 0.40 | -23.650 |
| Bromobenzene | 0.25 | 0.26 | 4.331 |
| 1-Octanol | 0.59 | 0.60 | 1.695 |
| Tetrahydrofuran | 0.22 | 0.22 | 3.530 |
Our evaluation determined that the Lee + Kesler estimation technique should not be used for perfluorocarbons. The technique's applicability rule shows this exclusion on lines 012 through 025. To summarize:, the code checks if there are only two types of elements in the chemical's molecular structure and if these two elements are carbon and fluorine.
- the code compiles the elements contained within the chemical's molecular structure (code lines 008 and 009)
- the code checks if there are only two types of element (code line 010)
- the positions of 'Fluorine' and 'Carbon' in the elements array are determined (code lines 012 through 014)
- if both fluorine and carbon are present in the array, i.e., their positions in the array are not equal to -1, then the chemical is a perfluorocabon and the technique is not applicable (code lines 016 through 026)
For non-perfluorocarbons, the average absolute error is 0.0288.
The following calculator demonstrates the use of the Lee + Kesler estimation method. Enter values for the input properties in the table to the right and then press the 'Estimate' 'button. Property estimates are generated by an online version of Cranium, Web Server Edition. The Web Server Edition of Cranium enables you to distribute physical property values and estimates via the internet. Please contact us if you would would like to learn more about how you organization can utilized the Web Server Edition of Cranium.
Calculation: enter values for the required physical properties in the units given in the 'Acentric Factor Estimation' table below. Then press the 'Estimate' button. A request will be sent to an online Web Server Edition of Cranium to perform the estimation. The resulting estimate will be displayed in the 'Estimated' table below. (Note that if you do not know values for the required input properties, draw your chemical's molecular structure in the editor to the right and press the editor's Estimate button. Cranium will estimate the required input property values.)
| Acentric Factor Estimation | ||
|---|---|---|
| Property | Value | Units |
| Boiling Point | K | |
| Critical Temperature | K | |
| Critical Pressure | atm | |
The physical properties entered above are added to a property estimation request which is sent to an instance of our Cranium, Web Server Edition software product running on a Microsoft Azure virtual machine. Cranium processes the request generating an estimated physical property value. This resulting value is then sent back to this webpage for display.
Click here to learn more about how you can use Cranium Web Server to distribute your company's physical property data, estimates, and knowledge throughout your organization or contact us for further details.
- Follow a step-by-step physical property estimation example
- Download and experiment with our Cranium Basic Edition
- Download and experiment with our Synapse Basic Edition
- Download and read through our Cranium brochure
- Download and read through our Synapse brochure
- View one of our demonstration videos