Today’s chemical products must not only possess superior performance but also low toxicity and environmental compatibility, while being safe and highly innovative. Satisfying all these often conflicting constraints is a challenge for product designers. Synapse is a software tool which greatly assists in the design of better chemical products.
Synapse is an advanced chemical product design software tool giving you a radically new approach for designing molecules and formulations that possess desired physical properties. You first enter constraints, such as the need to form an azeotrope with water, minimum solubility limits, maximum volatility and minimum flash point. Synapse then generates thousands of candidate molecules computationally assembling each candidate’s molecular structure atom by atom. Mixture formulations are similarly generated by choosing from hundreds of possible components and enumerating thousands of compositions. Synapse finally estimates the physical properties of each of these candidates and evaluates each design constraint identifying those candidates which satisfy all design constraints.
The goal of a chemical product design is to create a molecular structure or mixture formulation that possesses a desired set of chemical and physical properties. Thus the first step of any design involves identifying design constraints.
Synapse represents chemical constraints as limits on substructures. For example, many chemical products should be stable at ambient temperatures. Thus, we often use a maximum limit of zero on unstable substructure such as:
| -O-O- | >N-O- | -O-CO-O- | >N-N< | -CO-O-CO- |
Synapse will eliminate any candidate structure that does not satisfy a structural constraint.
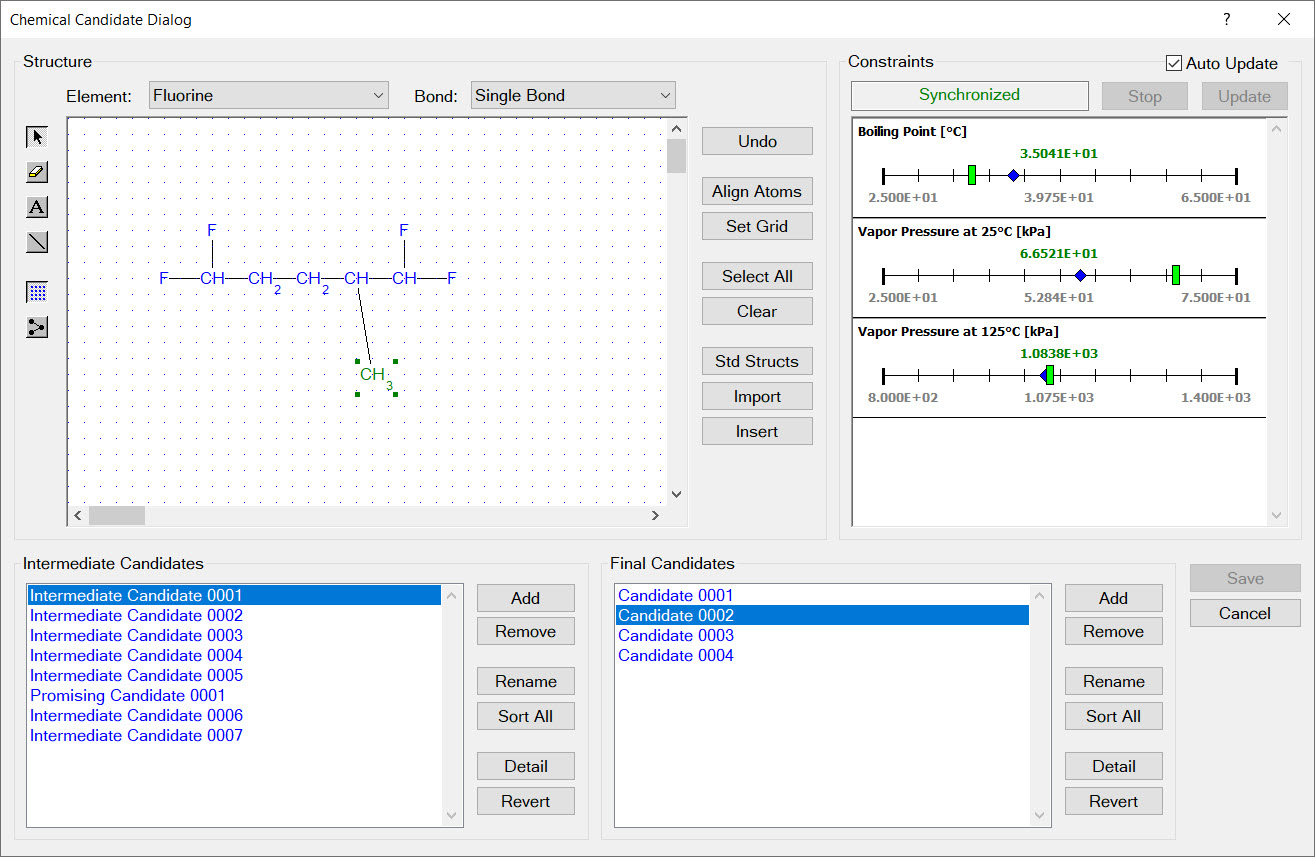
Design constraints are represented as ranges on physical properties. For example, the image to the left shows two design constraints:
353.15 K < boiling point < 393.15
800.0 kg/m3 < liquid density at 323.15 K < 1200.0 kg/m3
Synapse designs chemicals by assembling design groups into new molecular structures. Any set of groups can be used for a design. The selection of groups for new molecular structures can be guided by the user in a graphical design or automatically guided by the computer in a combinatorial design.
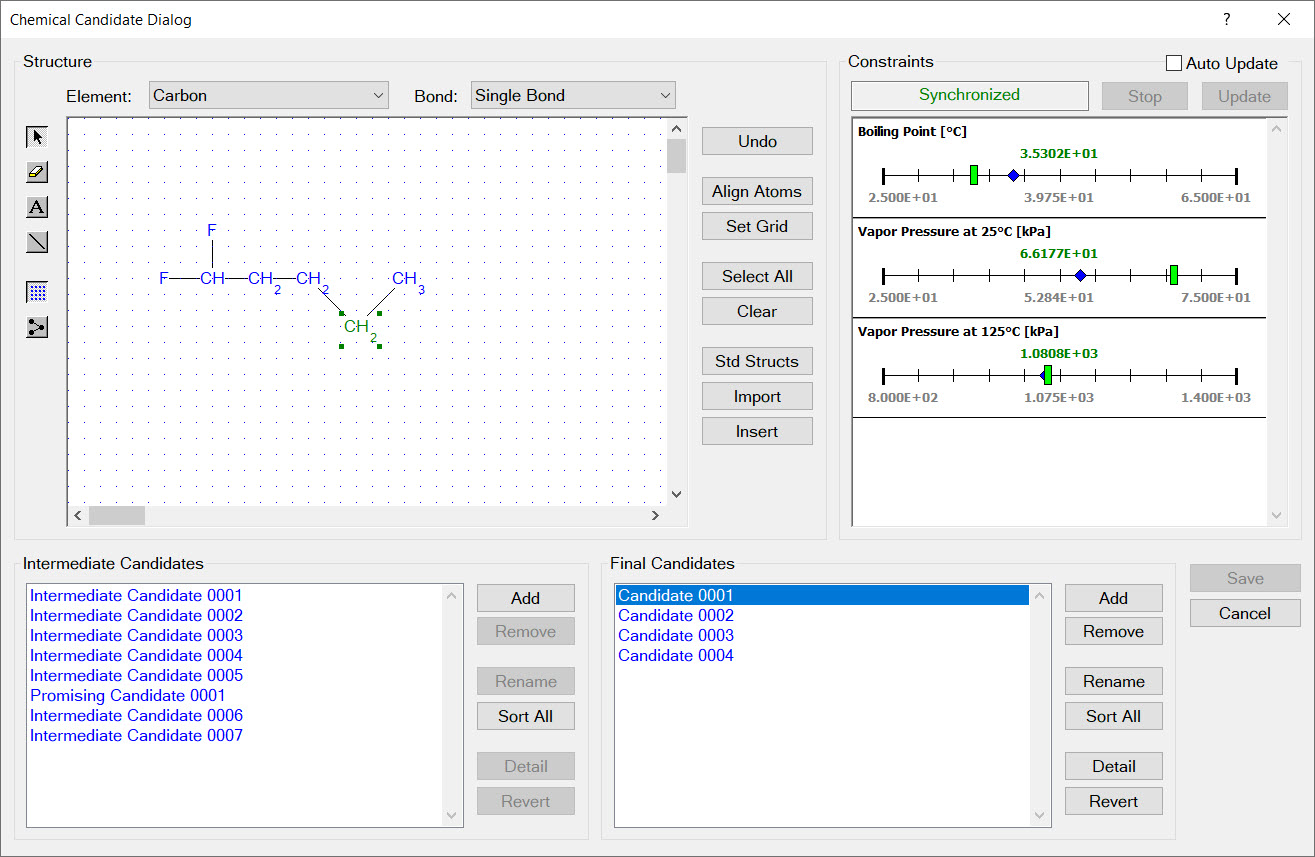
The image to the right shows that each design group has a set of limits imposed on its occurrence in new molecular structures. For example, every new molecular structure must contain at least two fluorine groups but no more than five fluorine groups.
In addition to limits on the occurrence of each design group in new molecular structures, each design must also contain limits on the total number of groups and total number of rings in new molecular structures. In the example shown in the image to the right, all newly designed molecular structures must contain between 4 and 8 groups and no rings.
Synapse’s graphical chemical design capabilities gives you complete control over the search for new chemical products. To design new chemicals you to simply draw candidate molecular structures. Synapse then estimates required physical properties, evaluates design constraints and presents the results graphically. Using Synapse’s graphical design you can investigate the effect of changing your current product’s structure or composition, identify conflicting design constraints and discover relationships between molecular structure and physical properties.
For example, you could use Synapse to design new chemicals based similar to ethyl levulinate that have applications as fragrance chemicals:
- you first enter constraints based on ethyl levulinate's physical properties
- you then draw ethyl levulinate's structure into the graphical design's edit control
- you then proceed to make structural modifications to the structure with the goal of satisfying the entered constraints
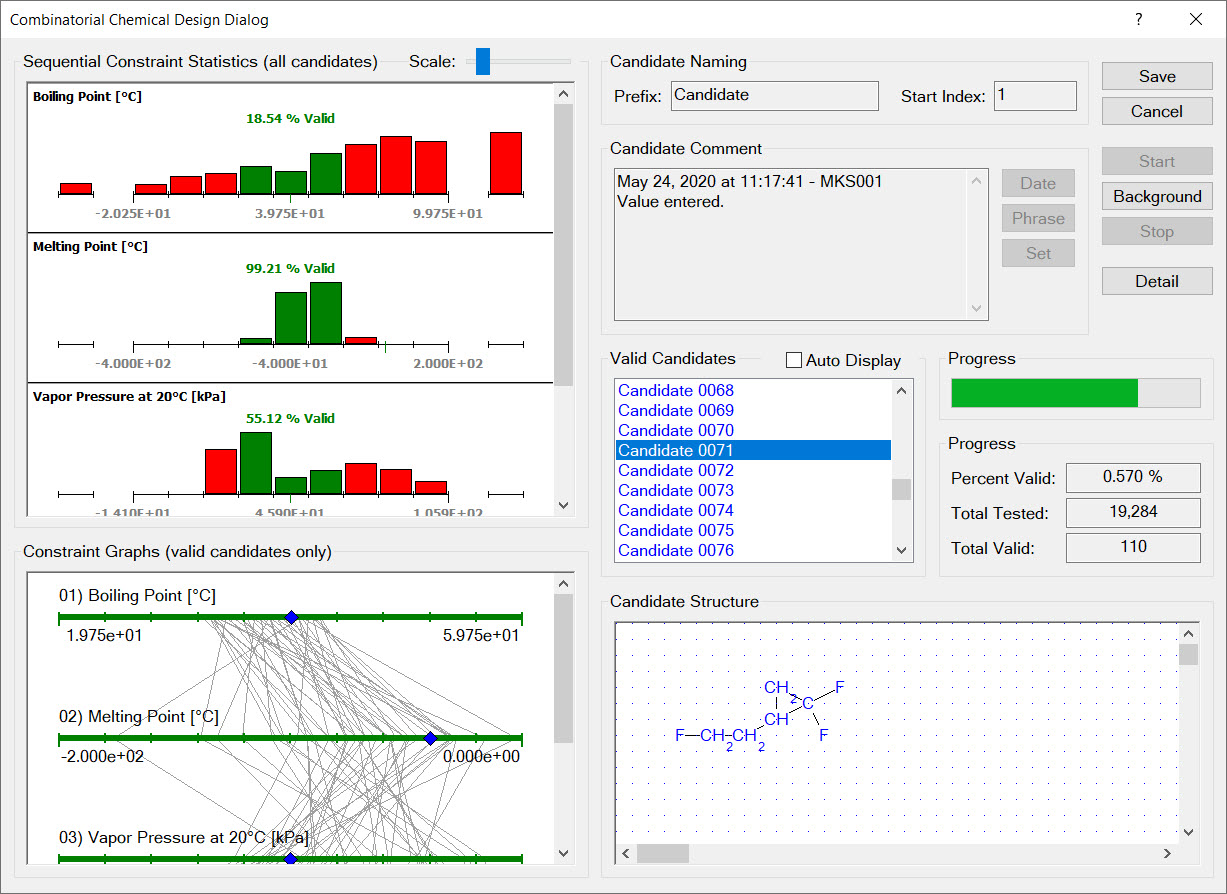
Synapse’s combinatorial chemical design capabilities enable you to automatically search through thousands of candidate molecular structures finding those that satisfy your design constraints. Synapse assembles each of candidate structure by connecting design groups in all possible combinations. Each candidate structure is examined to ensure it satisfies all substructure limit constraints. The physical properties of remaining candidates are estimated and used to evaluate the physical property design constraints.
A combinatorial chemical design is a powerful tool for exploring the space of possible chemical products. Because chemical designs are based on groups, you can add any group you wish to a design. For example, if you were looking into chemicals derived from levulinic acid you would create the group:
Synapse would then design candidate molecular structures by substituting design groups, in all possible combinations, for the free atoms (the [*] atoms).
Synapse's mixture designs are based on groups of ingredient chemicals called categories. A category represents a general set of chemicals. For example, an aircraft deicing fluid could have five categories: 1) water; 2) freezing point depressant; 3) surfactant; 4) thickener; 5) dye. The water category would contain a single chemical, i.e., water. The freezing point depressant category could contain several chemicals, e.g., 1,2-propylene glycol, 1-3 propylene glycol, ethanol, diethylene glycol, etc.
Synapse assembles mixtures from combinations of category chemicals. Each combination of chemicals is then assigned a composition using the input composition limits. The physical properties of this final mixture candidate are then estimated and used to evaluate each physical property design constraint.
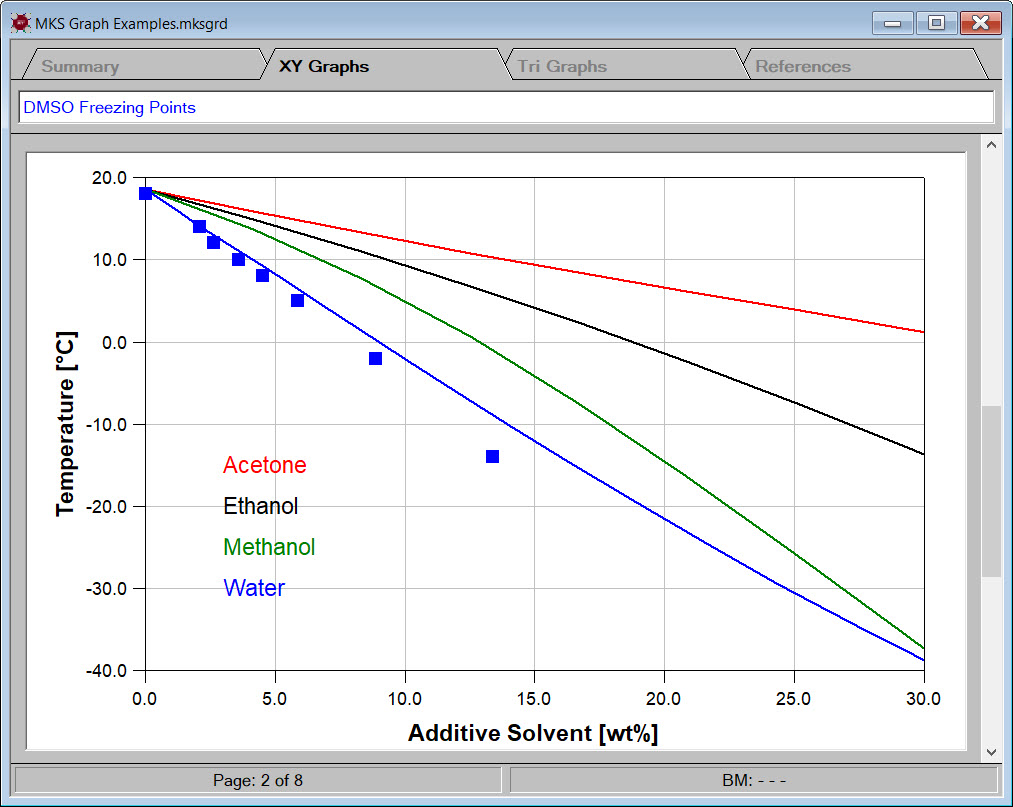
For example, DMSO is a good solvent for many applications. Unfortunately, DMSO's high melting point of 18.52C can sometimes result in the freezing of stored material. Adding a freezing point depressing additive solvent can significantly lower the freezing point.
Designing such low freezing point mixtures in Synapse begins by creating two ingredient categories, a DMSO category and an additive category. The DMSO category contains only one chemical, i.e., DMSO. The additive category contains several common solvents. The image to the left shows the solid-liquid equilibrium curves for some of the designed candidates.
| Some of the Physical Properties Estimated by Synapse | ||||
|---|---|---|---|---|
| Acentric Factor | Activity Coefficient | Aquatic Toxicity | Autoignition Temperature | Boiling Point |
| Bubble Point | Critical Pressure | Critical Temperature | Critical Volume | Densities |
| Diffusion Coefficients | Dew Point | Enthalpy of Formation | Enthalpy of Fusion | Enthalpy of Vaporization |
| Flash Point | Freezing Point | Fugacity Coefficient | Gibbs Energy of Formation | Heat Capacities |
| Henry's Constant | Lower Flammability Limit | Melting Point | Molecular Weight | Octanol-Water Partition |
| Refractive Index | Relative Permittivity | Solubility Parameter | Speed of Sound | Surface Tension |
| Thermal Conductivities | Upper Flammability Limit | Vapor Pressure | Viscosities | Water Solubility |
We are continually evaluating and adding new estimation techniques:
- we first compile the data needed to evaluate a new technique
- we code the technique into a knowledge base document using MKS's simple input language
- we use the analysis tools within Synapse to determine the applicability and accuracy of the technique
- we finally post the updated knowledge base on our website where our users can download the new data and new technique
| Property | Estimation Technique |
|---|---|
| Acentric Factor | AcF: Definition [MKS] |
| Acentric Factor | AcF: Lee + Kesler Relation [MKS] |
| Activity Coefficient, LLE - f(T,P,X) | ActC,LLE (T,P,X): UNIFAC Method [MKS] |
| Activity Coefficient, VLE - f(T,P,X) | ActC,VLE (T,P,X): DECHEMA, Margules - 500 mmHg [MKS] |
| Activity Coefficient, VLE - f(T,P,X) | ActC,VLE (T,P,X): Holmes + van Winkle, Margules - 500 mmHg [MKS] |
| Activity Coefficient, VLE - f(T,P,X) | ActC,VLE (T,P,X): Holmes + van Winkle, Margules - 760 mmHg [MKS] |
| Activity Coefficient, VLE - f(T,P,X) | ActC,VLE (T,P,X): Holmes + van Winkle, van Laar - 500 mmHg [MKS 01] |
| Activity Coefficient, VLE - f(T,P,X) | ActC,VLE (T,P,X): Holmes + van Winkle, van Laar - 760 mmHg [MKS] |
| Activity Coefficient, VLE - f(T,P,X) | ActC,VLE (T,P,X): Holmes + van Winkle, Wilson - 760 mmHg [MKS] |
| Activity Coefficient, VLE - f(T,P,X) | ActC,VLE (T,P,X): Modified UNIFAC (Dortmund) Method [MKS] |
| Activity Coefficient, VLE - f(T,P,X) | ActC,VLE (T,P,X): MOSCED [MKS] |
| Activity Coefficient, VLE - f(T,P,X) | ActC,VLE (T,P,X): UNIFAC Method [MKS] |
| Autoignition Temperature | AIT: Chen + Liaw + Kuo Method [MKS] |
| Boiling Point | Tb: Antoine Equation - PGL 2001 [MKS] |
| Boiling Point | Tb: Joback Method [MKS] |
| Boiling Point | Tb: Stein + Brown Method [MKS] |
| Boiling Point - f(X) | Tb (X): Gamma-Ideal Method [MKS] |
| Critical Compressibility | Zc: Definition [MKS] |
| Critical Pressure | Pc: Joback Method [MKS] |
| Critical Pressure | Pc: Lydersen Method [MKS] |
| Critical Pressure | Pc: Myers + Danner Technique [MKS] |
| Critical Pressure | Pc: Vapor Pressure Extrapolation [MKS] |
| Critical Pressure | Pc: Wilson + Jasperson Method [MKS] |
| Critical Pressure - f(X) | Pc (X): Chueh + Prausnitz Method [MKS] |
| Critical Pressure - f(X) | Pc (X): Kreglewski + Kay Method [MKS] |
| Critical Temperature | Tc: Fedors Technique [MKS] |
| Critical Temperature | Tc: Joback Method [MKS] |
| Critical Temperature | Tc: Klincewicz Method [MKS] |
| Critical Temperature | Tc: Lydersen Method [MKS] |
| Critical Temperature | Tc: Myers + Danner Technique [MKS] |
| Critical Temperature | Tc: Tu Method [MKS] |
| Critical Temperature | Tc: Wilson + Jasperson Method - First Order [MKS] |
| Critical Temperature - f(X) | Tc (X): Chueh + Prausnitz Method [MKS] |
| Critical Temperature - f(X) | Tc (X): Li Technique [MKS] |
| Critical Volume | Vc: Ambrose Method [MKS] |
| Critical Volume | Vc: Joback Method [MKS] |
| Critical Volume | Vc: Lydersen Method [MKS] |
| Critical Volume - f(X) | Vc (X): Chueh + Prausnitz Method [MKS] |
| Critical Volume - f(X) | Vc (X): Li + Kiran + Lydersen Method [MKS] |
| Density, Liquid - f(T) | Den,l (T): Bhirud Technique [MKS] |
| Density, Liquid - f(T) | Den,l (T): Dippr Equation 105 [MKS] |
| Density, Liquid - f(T) | Den,l (T): GCVol Method [MKS] |
| Density, Liquid - f(T) | Den,l (T): Hankinson + Thomson [MKS] |
| Density, Liquid - f(T) | Den,l (T): IAPWS Formula 1995 [MKS] |
| Density, Liquid - f(T) | Den,l (T): Modified Rackett Equation [MKS] |
| Density, Liquid - f(T) | Den,l (T): Peng + Robinson EOS [MKS] |
| Density, Liquid - f(T) | Den,l (T): Rackett Equation [MKS] |
| Density, Liquid - f(T) | Den,l (T): Redlich + Kwong EOS [MKS] |
| Density, Liquid - f(T) | Den,l (T): Soave + Redlich + Kwong EOS [MKS] |
| Density, Liquid - f(T) | Den,l (T): van der Waals EOS [MKS] |
| Density, Liquid - f(T,P) | Den,l (T,P): Peng + Robinson EOS [MKS] |
| Density, Liquid - f(T,P) | Den,l (T,P): Redlich + Kwong EOS [MKS] |
| Density, Liquid - f(T,P) | Den,l (T,P): Soave + Redlich + Kwong EOS [MKS] |
| Density, Liquid - f(T,P) | Den,l (T,P): Thomas + Brobst + Hankinson Method [MKS] |
| Density, Liquid - f(T,P) | Den,l (T,P): van der Waals EOS [MKS] |
| Density, Liquid - f(T,P,X) | Den,l (T,P,X): Peng + Robinson EOS [MKS] |
| Density, Liquid - f(T,P,X) | Den,l (T,P,X): Soave + Redlich + Kwong EOS [MKS] |
| Density, Liquid - f(T,X) | Den,l (T,X): Hankinson + Thomson [MKS] |
| Density, Liquid - f(T,X) | Den,l (T,X): Spencer + Danner Method [MKS] |
| Density, Vapor - f(T,P) | Den,v (T,P): Ideal Gas Law [MKS] |
| Density, Vapor - f(T,P) | Den,v (T,P): Peng + Robinson EOS [MKS] |
| Density, Vapor - f(T,P) | Den,v (T,P): Redlich + Kwong EOS [MKS] |
| Density, Vapor - f(T,P) | Den,v (T,P): Soave + Redlich + Kwong EOS [MKS] |
| Density, Vapor - f(T,P) | Den,v (T,P): van der Waals EOS [MKS] |
| Density, Vapor - f(T,P,X) | Den,v (T,P,X): Peng + Robinson EOS [MKS] |
| Density, Vapor - f(T,P,X) | Den,v (T,P,X): Soave + Redlich + Kwong EOS [MKS] |
| Diffusion Coefficient, Vapor - f(T,P,X) | DiffC,v (T,P,X): Chapman + Enskog Method [MKS] |
| Enthalpy of Combustion at 298K | Hc,298: Enthalpy Difference Calculation [MKS] |
| Enthalpy of Formation, Liquid at 298K | Hf,l,298: Vapor Estimate Adjustment [MKS] |
| Enthalpy of Formation, Vapor at 298K | Hf,v,298: Joback Method [MKS] |
| Enthalpy of Fusion at Tm | Hm,tm: Joback Method [MKS] |
| Enthalpy of Vaporization - f(T) | Hv (T): Dippr Equation 106 [MKS] |
| Enthalpy of Vaporization - f(T) | Hv (T): Pitzer Correlation [MKS] |
| Enthalpy of Vaporization - f(T) | Hv (T): Tu + Liu Method [MKS] |
| Enthalpy of Vaporization - f(T) | Hv (T): Watson Relation [MKS] |
| Enthalpy of Vaporization at Tb | Hv,tb: Chen Method [MKS] |
| Enthalpy of Vaporization at Tb | Hv,tb: Joback Method [MKS] |
| Enthalpy of Vaporization at Tb | Hv,tb: Riedel Method [MKS] |
| Enthalpy of Vaporization at Tb | Hv,tb: Vetere Method [MKS] |
| Enthalpy, Liquid - f(T) | H,l (T): Trapezoid Integration Method [MKS] |
| Enthalpy, Liquid - f(T,P) | H,l (T,P): Trapezoid Integration Method [MKS] |
| Enthalpy, Vapor - f(T) | H,v (T): Trapezoid Integration Method [MKS] |
| Entropy, Liquid - f(T) | S,l (T): Trapezoid Integration Method [MKS] |
| Entropy, Vapor - f(T) | S,v (T): Trapezoid Integration Method [MKS] |
| Flammability Limit, Lower | LFL: Seaton Method [MKS] |
| Flammability Limit, Lower | LFL: Shebeko Atom Technique [MKS] |
| Flammability Limit, Lower | LFL: Shebeko Modified Technique [MKS] |
| Flammability Limit, Lower - f(X) | LFL (X): Le Chatelier Method [MKS] |
| Flammability Limit, Upper | UFL: High + Danner Method [MKS] |
| Flammability Limit, Upper | UFL: Seaton Method [MKS] |
| Flammability Limit, Upper - f(X) | UFL (X): Le Chatelier Method [MKS] |
| Flash Point, Closed Cup | Tf,cc: Affens Method [MKS] |
| Flash Point, Closed Cup | Tf,cc: Butler + Cooke + Lukk + Jameson Method [MKS] |
| Flash Point, Closed Cup | Tf,cc: Catoire + Naudet Method [MKS] |
| Flash Point, Closed Cup | Tf,cc: Hshieh Organics Method [MKS] |
| Flash Point, Closed Cup | Tf,cc: Patil Method [MKS] |
| Flash Point, Closed Cup | Tf,cc: Satyanarayana + Kakati Method [MKS] |
| Flash Point, Closed Cup - f(X) | Tf,cc (X): Catoire + Paulmier + Naudet Ideal Method [MKS] |
| Flash Point, Closed Cup - f(X) | Tf,cc (X): Catoire + Paulmier + Naudet Method [MKS] |
| Flash Point, Closed Cup - f(X) | Tf,cc (X): Liaw + Tang + Lai Method [MKS] |
| Fugacity Coefficient, Liquid - f(T,P) | Fug,l (T,P): Peng + Robinson EOS [MKS] |
| Fugacity Coefficient, Liquid - f(T,P,X) | Fug,l (T,P,X): Peng + Robinson EOS [MKS] |
| Fugacity Coefficient, Vapor - f(T,P) | Fug,v (T,P): Peng + Robinson EOS [MKS] |
| Fugacity Coefficient, Vapor - f(T,P,X) | Fug,v (T,P,X): Peng + Robinson EOS [MKS] |
| General Calculation | GenCalc: 2-Hydroxybenzoic acid solubility at 25°C [MKS] |
| General Calculation | GenCalc: Diffusion in Air (25C, 101kPa, cm2/sec) [MKS] |
| General Calculation | GenCalc: Hill Cross Sectional Area - [MKS] |
| General Calculation | GenCalc: Naphthalene solubility at 25°C [MKS] |
| General Calculation | GenCalc: Number of Oxygen Atoms [MKS] |
| General Calculation | GenCalc: Oxygen Balance Calculation [MKS] |
| General Calculation | GenCalc: Solubility in pyridine at 25°C [MKS] |
| General Calculation | GenCalc: Specific Gravity at 20°C [MKS] |
| General Calculation - f(X) | GenCalc (X): Binary Azeotrope Formed [MKS] |
| General Calculation - f(X) | GenCalc (X): Percentage of Wetted Surface Area - [MKS] |
| Gibbs Energy of Formation, Vapor at 298K | Gf,v,298: Joback Method [MKS] |
| Heat Capacity - Isobaric, Liquid - f(T) | Cp,l (T): Dippr Equation 100 [MKS] |
| Heat Capacity - Isobaric, Liquid - f(T) | Cp,l (T): IUPAC Cubic Splines [MKS] |
| Heat Capacity - Isobaric, Liquid - f(T) | Cp,l (T): Missenard Method [MKS] |
| Heat Capacity - Isobaric, Liquid - f(T) | Cp,l (T): Poling + Prausnitz + O'Connell CSP Method [MKS] |
| Heat Capacity - Isobaric, Liquid - f(T,X) | Cp,l (T,X): Ideal Molar Mixing Rule [MKS] |
| Heat Capacity - Isobaric, Liquid at 298K | Cp,l,298: Chickos + Acree Method [MKS] |
| Heat Capacity - Isobaric, Solid - f(T) | Cp,s (T): Goodman + Wilding + Oscarson + Rowley Method [MKS] |
| Heat Capacity - Isobaric, Solid at 298K | Cp,s,298: Chickos + Acree Method [MKS] |
| Heat Capacity - Isobaric, Solid at 298K | Cp,s,298: Hurst + Harrison [MKS] |
| Heat Capacity - Isobaric, Vapor - f(T) | Cp,v (T): Dippr Equation 107 [MKS] |
| Heat Capacity - Isobaric, Vapor - f(T) | Cp,v (T): Joback Method [MKS] |
| Heat Capacity - Isobaric, Vapor - f(T,X) | Cp,v (T,X): Ideal Molar Mixing Rule [MKS] |
| Heat Capacity - Isobaric, Vapor at 298K | Cp,v,298: Fixed Temperature Method [MKS] |
| Heat Capacity - Isobaric, Vapor at 298K | Cp,v,298: Joback Method [MKS] |
| Heat Capacity - Isometric, Vapor - f(T) | Cv,v (T): Ideal Gas Relation [MKS] |
| Henry's Constant (pc) in H2O - f(T) | Hpc (T): Penttilä + Dell'Era + Uusi-Kyyny + Alopaeus Method [MKS] |
| Henry's Constant (px) in H2O - f(T) | Hpx (T): Carroll + Slupsky + Mather Method [MKS] |
| Henry's Constant (px) in H2O - f(T) | Hpx (T): Fernández-Prini + Alvarez + Harvey Method [MKS] |
| LC50 96hr, Fathead Minnow | LC50,96hr,FatMn: Martin + Young Method [MKS] |
| log(Octanol/Water Partition Coefficient) | log P: Lin + Sandler Method [MKS] |
| Melting Point | Tm: Constantinou + Gani First Order Method [MKS] |
| Melting Point | Tm: Joback Method [MKS] |
| Molecular Weight | Mw: Definition [MKS] |
| Molecular Weight - f(X) | Mw (X): Definition [MKS] |
| Refractive Index, Liquid at 293K | RI,l: Lorentz + Lorenz Equation [MKS] |
| SLE, Liquidus Temperature - f(P,X) | LiqPtTmp (P,X): Gamma VLE Eutectic Method [MKS] |
| SLE, Liquidus Temperature - f(P,X) | LiqPtTmp (P,X): Ideal Eutectic Model [MKS] |
| Solubility Parameter, Dispersive | SP,d: Stefanis + Panayiotou Method, First Order [MKS] |
| Solubility Parameter, Dispersive - f(X) | SP,d (X): Ideal Volume Fraction Average [MKS] |
| Solubility Parameter, Hydrogen Bonding | SP,h: Stefanis + Panayiotou Method, First Order - Std [MKS] |
| Solubility Parameter, Hydrogen Bonding - f(X) | SP,h (X): Ideal Volume Fraction Average [MKS] |
| Solubility Parameter, Polar | SP,p: Hansen + Beerbower Method [MKS] |
| Solubility Parameter, Polar | SP,p: Stefanis + Panayiotou Method, First Order - Std [MKS] |
| Solubility Parameter, Polar - f(X) | SP,p (X): Ideal Volume Fraction Average [MKS] |
| Solubility Parameter, Total | SP,t: Definition [MKS] |
| Solubility Parameter, Total | SP,t: Fedors Technique [MKS] |
| Solubility Parameter, Total | SP,t: Three Term Definition - Data [MKS] |
| Solubility Parameter, Total | SP,t: Three Term Definition [MKS] |
| Speed of Sound, Liquid - f(T) | SpSnd,l (T): Peng + Robinson EOS [MKS] |
| Speed of Sound, Vapor - f(T,P) | SpSnd,v (T,P): Peng + Robinson EOS [MKS] |
| Surface Tension, Liquid - f(T) | SurfTn,l (T): Brock + Bird Method [MKS] |
| Surface Tension, Liquid - f(T) | SurfTn,l (T): Dippr Equation 106 [MKS] |
| Surface Tension, Liquid - f(T) | SurfTn,l (T): Sastri + Rao Method [MKS] |
| Surface Tension, Liquid - f(T) | SurfTn,l (T): Somayajulu [MKS] |
| Surface Tension, Liquid - f(T,X) | SurfTn,l (T,X): Molar Average [MKS] |
| Thermal Conductivity, Liquid - f(T) | ThrmCnd,l (T): Dippr Equation 100 [MKS] |
| Thermal Conductivity, Liquid - f(T) | ThrmCnd,l (T): Mallan + Michaelian + Lockhart Method [MKS] |
| Thermal Conductivity, Liquid - f(T) | ThrmCnd,l (T): Missenard + Riedel Method [MKS] |
| Thermal Conductivity, Liquid - f(T) | ThrmCnd,l (T): Sastri + Rao Method [MKS] |
| Thermal Conductivity, Liquid - f(T) | ThrmCnd,l (T): Sato + Riedel Method [MKS] |
| Thermal Conductivity, Liquid - f(T,X) | ThrmCnd,l (T,X): Filippov Equation [MKS] |
| Thermal Conductivity, Liquid - f(T,X) | ThrmCnd,l (T,X): Jamieson + Irving + Tudhope Correlation [MKS] |
| Thermal Conductivity, Liquid - f(T,X) | ThrmCnd,l (T,X): Power Law Relation [MKS] |
| Thermal Conductivity, Vapor - f(T) | ThrmCnd,v (T): Dippr Equation 102 [MKS] |
| Thermal Conductivity, Vapor - f(T) | ThrmCnd,v (T): Eucken Correlation [MKS] |
| Thermal Conductivity, Vapor - f(T) | ThrmCnd,v (T): Modified Eucken Correlation [MKS] |
| Thermal Conductivity, Vapor - f(T) | ThrmCnd,v (T): Stiel + Thodos Method [MKS] |
| Thermal Conductivity, Vapor - f(T,P) | ThrmCnd,v (T,P): Stiel + Thodos High Pressure Method [MKS] |
| Triple Point, Pressure | TrpPtPrs: Liquid Vapor Pressure Method [MKS] |
| Triple Point, Pressure | TrpPtPrs: Solid Vapor Pressure Method [MKS] |
| Triple Point, Temperature | TrpPtTmp: Melting Point Technique |
| Vapor Pressure, Liquid - f(T) | Pvp,l (T): Ambrose + Walton Method [MKS] |
| Vapor Pressure, Liquid - f(T) | Pvp,l (T): Antoine Equation - PGL 2001 [MKS] |
| Vapor Pressure, Liquid - f(T) | Pvp,l (T): Dippr Equation 101 [MKS] |
| Vapor Pressure, Liquid - f(T) | Pvp,l (T): Gómez-Nieto + Thodos Equation [MKS] |
| Vapor Pressure, Liquid - f(T) | Pvp,l (T): IAPWS Formula 1995 [MKS] |
| Vapor Pressure, Liquid - f(T) | Pvp,l (T): Lee + Kesler Equation [MKS] |
| Vapor Pressure, Liquid - f(T) | Pvp,l (T): Riedel + Plank + Miller Equation [MKS] |
| Vapor Pressure, Solid - f(T) | Pvp,s (T): Jones Method [MKS] |
| Viscosity, Liquid - f(T) | Visc,l (T): Dippr Equation 101 [MKS] |
| Viscosity, Liquid - f(T) | Visc,l (T): Joback Method [MKS] |
| Viscosity, Liquid - f(T) | Visc,l (T): Orrick + Erbar Method [MKS] |
| Viscosity, Liquid - f(T) | Visc,l (T): Przezdziecki + Sridhar Method [MKS] |
| Viscosity, Liquid - f(T,P) | Visc,l (T,P): Lucas Method [MKS] |
| Viscosity, Liquid - f(T,X) | Visc,l (T,X): Arrhenius Equation [MKS] |
| Viscosity, Liquid - f(T,X) | Visc,l (T,X): Kendall + Monroe Relation [MKS] |
| Viscosity, Vapor - f(T) | Visc,v (T): Dippr Equation 102 [MKS] |
| Viscosity, Vapor - f(T) | Visc,v (T): Lucas Method [MKS] |
| Viscosity, Vapor - f(T) | Visc,v (T): Reichenberg Technique [MKS] |
| Viscosity, Vapor - f(T) | Visc,v (T): Yoon + Thodos Method [MKS] |
| Viscosity, Vapor - f(T,P) | Visc,v (T,P): Reichenberg Method [MKS] |
| Viscosity, Vapor - f(T,X) | Visc,v (T,X): Wilke Equation [MKS] |
| VLE, Bubble Pressure - f(T,X) | BubPtPrs (T,X): Gamma-Ideal Method [MKS] |
| VLE, Bubble Pressure - f(T,X) | BubPtPrs (T,X): Ideal-Ideal Method [MKS] |
| VLE, Bubble Pressure - f(T,X) | BubPtPrs (T,X): Phi-Phi Method [MKS] |
| VLE, Bubble Temperature - f(P,X) | BubPtTmp (P,X): Gamma-Ideal Method [MKS] |
| VLE, Bubble Temperature - f(P,X) | BubPtTmp (P,X): Ideal-Ideal Method [MKS] |
| VLE, Bubble Temperature - f(P,X) | BubPtTmp (P,X): Phi-Phi Method [MKS] |
| VLE, Dew Pressure - f(T,X) | DewPtPrs (T,X): Gamma-Ideal Method [MKS] |
| VLE, Dew Pressure - f(T,X) | DewPtPrs (T,X): Ideal-Ideal Method [MKS] |
| VLE, Dew Pressure - f(T,X) | DewPtPrs (T,X): Phi-Phi Method [MKS] |
| VLE, Dew Temperature - f(P,X) | DewPtTmp (P,X): Gamma-Ideal Method [MKS] |
| VLE, Dew Temperature - f(P,X) | DewPtTmp (P,X): Ideal-Ideal Method [MKS] |
| VLE, Dew Temperature - f(P,X) | DewPtTmp (P,X): Phi-Phi Method [MKS] |
Synapse come in four editions:
- Professional Edition: the Professional Edition provides an extensive windows interface, physical property data management, physical property estimation, chemical and mixture design and selection, and capabilities for graphing, reporting, and machine learning. The defining feature of the Professional Edition is that it enables you to create new documents, add new property data, enter new chemical structures, add new references, write new estimation techniques, and enter new designs and selections.
- Basic Edition: the Basic Edition also provides an extensive windows interface, physical property data management, physical property estimation, chemical and mixture design and selection, and capabilities for graphing, reporting, and machine learning. However, unlike the Professional Edition, none of your changes or additions can be saved with the Basic Edition. This makes the Basic Edition a great choice for experimentation. You may download the Basic Edition from our website.
- WebServer Edition: the WebServer Edition manages physical property data, provides physical property estimates, and has capabilities for graphing, reporting, and exporting. However. the WebServer Edition’s key capability is that it provides access to these data, estimates, and graphs via API requests over the Internet.
- Component Edition: The Component Edition manages physical property data, provides physical property estimates, and has capabilities for graphing, reporting, and exporting. However, the Component Edition’s key capability is that these data management and property estimation capabilities can be accessed by other Windows applications via a COM interface.